Nitriding
Nitriding is a similar process to carburizing. At elevated temperatures, a nitrogen rich (typically ammonia, NH3) gas is pumped into the furnace chamber. The ammonia disassociates upon contact with the metal into nitrogen (N) and hydrogen. The nitrogen then diffuses onto the surface of the material creating a nitride layer. This process produces a thin, hard layer on the surface of the steel that is more wear resistant than steel that has not been treated. This type of process has been used in the past to enhance the properties of shafting equipment, springs and piston rings, to name a few. Commercial nitriding typically takes place around 900°F but can reach temperatures up to 1100°F.
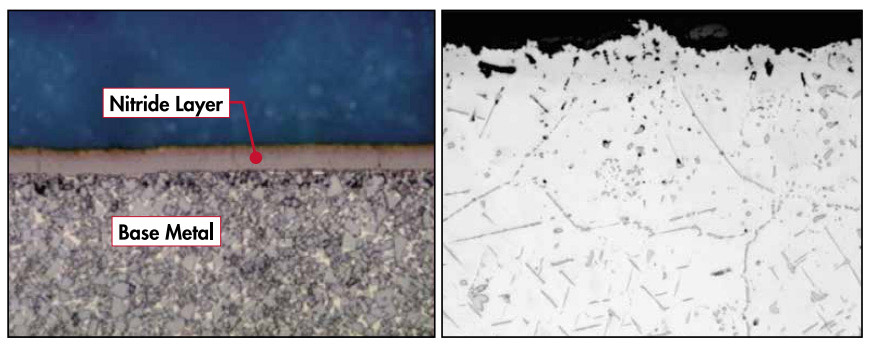
The picture below (left) shows an image of a piece of metal that has gone through the nitriding process. The gold color noticeable at the top of the metal itself is the nitrogen infused layer that is the product of the nitriding process. This allows for the surface to be hard and wear resistant while the base metal retains its’ tough, ductile properties beneath. The microstructure on the right shows what happens to high temperature grades like alloy 600 and RA330® in nitriding environments. Instead of a passive layer forming on the surface, nitrogen penetrates into the base metal reacting with the chromium to form chromium nitrides that look like needles on 60° angles. These nitrides embrittle the alloys instead of making them more wear resistant.
Carbo-Nitriding
Carbo-nitriding is a process carried out in an atmosphere containing both carbon and nitrogen. Temperatures are usually higher, around 1300-1650°F, than those used for nitriding, but lower than the carburizing process. The amount of time that a metal is in a carbo-nitriding atmosphere is usually shorter than the other processes by themselves.
The life of the alloy fixturing in a carbo-nitriding application cannot be expected to equal the life of a fixture in a straight carburizing environment for two reasons. The first reason is that the effect of the combination of both carbon and nitrogen embrittlement is more drastic than just one or the other. The second reason is that the cycles for carbo-nitriding are not as long. In this application, the life of the fixturing is not based on the total amount of time that an alloy sees this environment. It is based on the number of cycles. Thermal fatigue cracking gradually develops and grows with each cycle. A part in a carbo-nitriding environment will receive significantly more cycles in a given length of time than if it were in a carburizing application; and its life will be shortened accordingly.
Rolled Alloys carries alloys that are used for fixturing and maintaining the nitriding and carbo-nitriding furnaces. Commercial processes typically use alloy 600 for its’ low chromium and high nickel contents. Often, RA330 is substituted into the process in place of alloy 600 due to its’ lower cost. While RA330 is typically inferior to alloy 600 for this process, the life to cost ratio tends to make it a very viable option.