phosphoric acid
Phosphoric acid is the third largest commodity mineral acid, following behind sulfuric and nitric. There are two basic routes to production – wet process and electric furnace process. The bulk of the product is produced by the wet process method and is consumed largely in the production of fertilizer. Wet process acid typically contains impurities of sulfates, fluorides and fluosilicates. The purer electric furnace process acid is primarily used for the production of soaps, water conditioning, metal cleaning and as a food/beverage additive.
Phosphoric acid generally acts as a reducing acid. The presence of fluoride contaminants or oxidizing ions can radically alter a material’s performance. Aluminum alloys offer resistance up to about 20% concentration and 65°C (149°F). However, metal ions or halide contamination adversely affects performance. Copper alloys have been used in deaerated applications where oxidizing ions are absent.
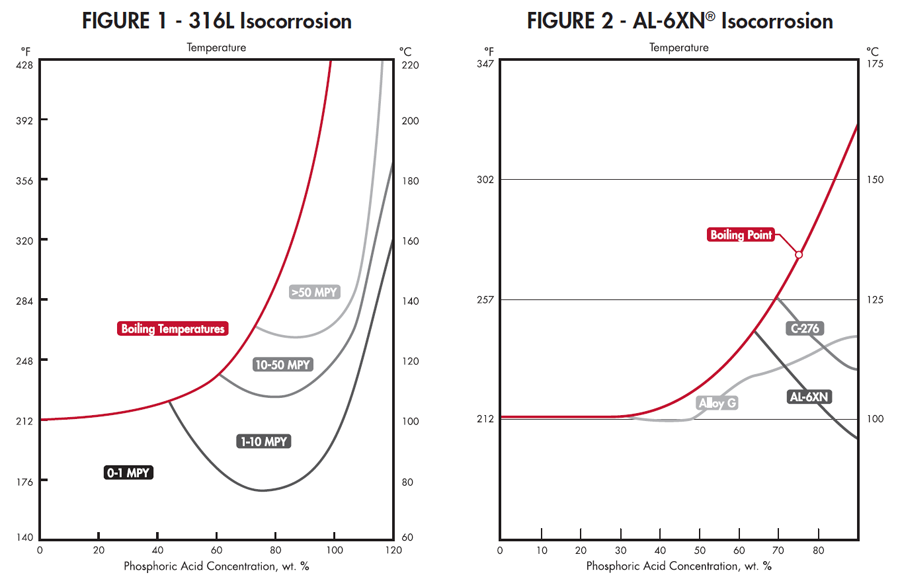
Most applications rely on stainless steels or nickel alloys. Straight chromium grades are generally not suitable and the least resistant grade of stainless commonly used is 316L. An isocorrosion diagram for 316L in phosphoric is shown (above left) in Figure 1. Higher levels of chromium and molybdenum increase phosphoric acid resistance. Alloys 317L and AL-6XN® perform better than 316L or Alloy 20. An isocorrosion diagram for AL-6XN Alloy is shown (above right) in Figure 2. It has also been noted that copper ions act as an inhibitor so alloys with copper additions tend to have a slight advantage. Alloy G and its variants, such as G-3 and G-30®, have demonstrated good performance in concentrated wet process acid at elevated temperatures.
Alloys 200 and 400 are generally less resistant than copper alloys and are seldom used. Alloy B-2 (Ni-Mo) is resistant to all concentrations in pure acid, but is rapidly attacked when oxidizing ions are present. Ni-Cr-Mo type alloys that are more highly alloyed than the G alloys, such as 625 and C-276, are resistant in pure acid. While they can be useful in the presence of oxidizing ions, they do not offer an advantage over the G types.
Ti-Pd alloys, zirconium and tantalum may find limited use in pure phosphoric acid, but do not tolerate even small amounts of fluoride contamination.
Most applications rely on stainless steels or nickel alloys. Straight chromium grades are generally not suitable and the least resistant grade of stainless commonly used is 316L. An isocorrosion diagram for 316L in phosphoric is shown (above left) in Figure 1. Higher levels of chromium and molybdenum increase phosphoric acid resistance. Alloys 317L and AL-6XN® perform better than 316L or Alloy 20. An isocorrosion diagram for AL-6XN Alloy is shown (above right) in Figure 2. It has also been noted that copper ions act as an inhibitor so alloys with copper additions tend to have a slight advantage. Alloy G and its variants, such as G-3 and G-30®, have demonstrated good performance in concentrated wet process acid at elevated temperatures.
Alloys 200 and 400 are generally less resistant than copper alloys and are seldom used. Alloy B-2 (Ni-Mo) is resistant to all concentrations in pure acid, but is rapidly attacked when oxidizing ions are present. Ni-Cr-Mo type alloys that are more highly alloyed than the G alloys, such as 625 and C-276, are resistant in pure acid. While they can be useful in the presence of oxidizing ions, they do not offer an advantage over the G types.
Ti-Pd alloys, zirconium and tantalum may find limited use in pure phosphoric acid, but do not tolerate even small amounts of fluoride contamination.